Abstract
Background: Previous studies suggest that survival improves when patients (pts) with rare cancers are treated at specialty centers. Majority of MDS cases in the US are diagnosed in the communities. There is a perception that outcome of higher-risk MDS pts treated in communities is inferior when compared to specialty centers for several reasons, including the diagnostic complexities of MDS leading to treatment delays, complicated criteria for assessing treatment response, limited availability of clinical trials when hypomethylating agents (HMAs) fail and limited access to transplant referrals, among others. Integrating data acquired from academic institutions that form the U.S. MDS Clinical Research Consortium (CRC) and the Surveillance, Epidemiology, and End Results (SEER) Program, we evaluated the extent to which the databases provide evidence to suggest that an average HMA-treated patient experienced differential survival after receiving care at a specialty center when compared to care in the community. The study focused on MDS pts treated with HMAs.
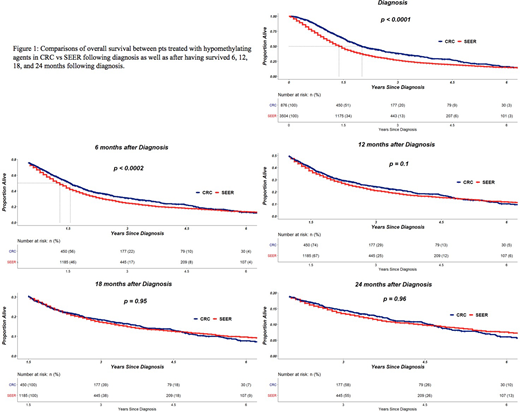
Methods: Outcomes of pts in SEER represents outcomes in the 'real world'. SEER MDS pts "treated with chemotherapy" most likely refers to receipt of HMAs. We identified all MDS patients (ICD-O3 in 9980 to 9989) who received chemotherapy and were diagnosed with MDS within the time interval observed in the CRC database. Using the SEER subset, CRC MDS pts treated with HMAs were matched 1:4 to commensurate SEER MDS pts by sex, age and year of diagnosis. Overall survival (OS) was estimated by the Kaplan-Meier method and evaluated for association with clinical source using Cox proportional hazard regression with two-sided Wald test adjusted for age at diagnosis. The regression analysis was applied to estimate the hazard ratio (HR) for OS over the entire risk duration (from diagnosis to death) as well as conditionally for pt subsets that had survived 6, 12, 18, and 24 months following diagnosis, respectively. This analysis strategy acknowledges the potential for unobserved heterogeneity not accounted for by the matching criteria (number of regimens received and whether pts underwent allogeneic hematopoietic cell transplantation) with respect treatment and baseline prognosis as well as enables estimation of time-varying benefit over time. Typically for most MDS pts treated with HMAs, the duration of response ranges from 6 months to 2 years (yrs), and hence, we chose this time frame for analysis.
Results: Of 5461 MDS cases diagnosed in SEER between 2006-2015 and treated with chemotherapy, 3504 were matched with CRC (876) MDS cases by sex, age and year of diagnosis. The resultant Kaplan-Meier curves are shown in Figure 1 for all matched pts following diagnosis as well as for subsets of pts that survived landmark time points. A total of 24% of HMA-treated pts died within the first 6 months from time of diagnosis. Roughly 50%, 31%, and 19% were alive at 12, 18, and 24 months following diagnosis, respectively. When estimating the entire duration of follow-up, a significant reduction in the rate of death was evident for CRC when compared to SEER (HR=0.78, p<0.0001). Additionally, improvement was estimated for CRC treated pts surviving at least 6 months from diagnosis (HR=0.82, p<0.0002). For pts surviving at least 12 months (HR=0.90, p=0.1), 18 months (HR=1.01, p=0.94), and 24 months (HR=1.00, p=0.96), the databases lacked statistical evidence to suggest that CRC offered a survival advantage when compared to SEER.
Conclusions: In matched analysis, MDS pts treated with HMAs at CRC centers have significantly better survival in the first year following diagnosis compared to SEER. The lowered risk of first year mortality following MDS diagnosis in pts treated with HMA at CRC compared to SEER is likely the result of earlier detection, earlier time to treatment, selection bias for healthier pts, and availability of clinical trials. Conditional survival probabilities beyond 12 months are comparable between real-world and specialty centers, an effect that most likely reflects the natural history of disease progression, loss of response to HMAs or lack of effective therapies beyond HMAs. These findings emphasize the need to factor in real world data in designing rigorous clinical trials to include mortality reduction beyond 12 months among several study end points to more comprehensively assess treatment effectiveness.
Mukherjee:LEK Consulting: Consultancy, Honoraria; Takeda: Membership on an entity's Board of Directors or advisory committees; BioPharm Communications: Consultancy; Projects in Knowledge: Honoraria; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squib: Honoraria, Speakers Bureau; Pfizer: Honoraria; Aplastic Anemia & MDS International Foundation in Joint Partnership with Cleveland Clinic Taussig Cancer Institute: Honoraria. Komrokji:Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding. Roboz:Argenx: Consultancy; Pfizer: Consultancy; Cellectis: Research Funding; Sandoz: Consultancy; Roche/Genentech: Consultancy; Celgene Corporation: Consultancy; Otsuka: Consultancy; Astex Pharmaceuticals: Consultancy; Eisai: Consultancy; Cellectis: Research Funding; Pfizer: Consultancy; Jazz Pharmaceuticals: Consultancy; Sandoz: Consultancy; Orsenix: Consultancy; Celgene Corporation: Consultancy; Aphivena Therapeutics: Consultancy; Aphivena Therapeutics: Consultancy; Roche/Genentech: Consultancy; AbbVie: Consultancy; Daiichi Sankyo: Consultancy; Novartis: Consultancy; Janssen Pharmaceuticals: Consultancy; Celltrion: Consultancy; Otsuka: Consultancy; Eisai: Consultancy; Janssen Pharmaceuticals: Consultancy; Argenx: Consultancy; Daiichi Sankyo: Consultancy; Orsenix: Consultancy; Novartis: Consultancy; AbbVie: Consultancy; Jazz Pharmaceuticals: Consultancy; Bayer: Consultancy; Bayer: Consultancy; Astex Pharmaceuticals: Consultancy; Celltrion: Consultancy. Maciejewski:Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Ra Pharmaceuticals, Inc: Consultancy; Ra Pharmaceuticals, Inc: Consultancy; Apellis Pharmaceuticals: Consultancy; Apellis Pharmaceuticals: Consultancy; Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Nazha:MEI: Consultancy. Sekeres:Opsona: Membership on an entity's Board of Directors or advisory committees; Opsona: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal